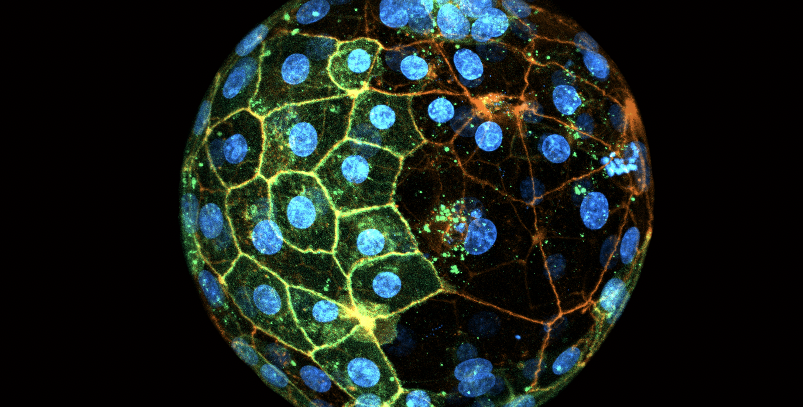
A human blastocyst expressing GFP in some cells (green), and stained to reveal the cell membranes (orange) and nuclei (blue). Labelling with GFP allowed the authors to reveal that only one 2-cell stage blastomere contributes most of the cells to the future body. Credit: Sergi Junyent Espinosa
Though over 8 million babies have been born through in vitro fertilization (IVF), 70 percent of IVF implantations fail. As IVF is becoming a more common route to pregnancy in cases of infertility, there is a need for better understanding of embryonic development at this early stage.
Researchers in the laboratory of Caltech’s Magdalena Zernicka-Goetz, Bren Professor of Biology and Biological Engineering, study the biological processes underlying the earliest days of human development. Now, a new study from the Zernicka-Goetz lab demonstrates that when human embryos are composed of two cells, at just 1 day old, only one of these cells will create most of the fetal body cells in addition to placental cells, while the other will only create placental cells. The research changes the long-standing paradigm that the two cells at this stage both contribute equally to all parts of the developing embryo, suggesting that “specification”—the phenomenon of cells having specific individual roles—happens much earlier in development than previously believed.
The findings have implications for how embryos intended for IVF implantation are assessed for abnormalities.
“Often, in an IVF clinic, a few placental cells from the outside of the 6-day-old embryo will be selected for a genetic diagnosis to determine if they have chromosomal abnormalities,” says Zernicka-Goetz. “Our results show that, by extrapolation, those outside cells chosen are unlikely to be contributing to the fetal body. The genetic information from those cells may not be as informative as sampling the fetal cells themselves.”
A paper describing the research appears on May 13 in the journal Cell.
The 1-day-old human embryo is composed of just two cells, each called a blastomere. Using embryos donated for research by IVF clinics, the team labeled blastomeres with a colored dye, then used time-lapse imaging to watch as the cells divided over the course of six days. New cells carried the same color dye as their parent cell. Through this process, the team determined that fetal body cells exclusively originated from a single blastomere, while placental cells came from both.
“In addition to being valuable information for improving IVF, our study is part of a large body of research into evolutionary processes within the body,” says postdoctoral scholar Sergi Junyent, a co-first author on the new paper. “Studying how different cell lineages populate from original cells has implications for understanding what happens after mutations, how they lead to cancer, and so on.”
The paper is titled “The first two blastomeres contribute unequally to the human embryo.” Caltech’s Junyent and Maciej Meglicki of the University of Cambridge are co-first authors. Additional Caltech co-authors are undergraduate Ekta M. Patel, scientific assistant Clare Reynell, and postdoctoral scholar Dong-Yuan Chen. Other co-authors are Catherine King and Lisa Iwamoto-Stohl of the University of Cambridge; Roman Vetter and Dagmar Iber of ETH Zurich and the Swiss Institute of Bioinformatics; Rachel Mandelbaum, Patrizia Rubino, and Richard J. Paulson of USC; and Nabil Arrach of Progenesis Inc. Funding was provided by the Human Frontier Science Program, NOMIS Foundation, Wellcome Trust, Open Philanthropy Project, and Curci and Weston Heavens Foundations. Magdalena Zernicka-Goetz is an affiliated faculty member with the Tianqiao and Chrissy Chen Institute for Neuroscience at Caltech.


















